Want to learn more? Contact us today.

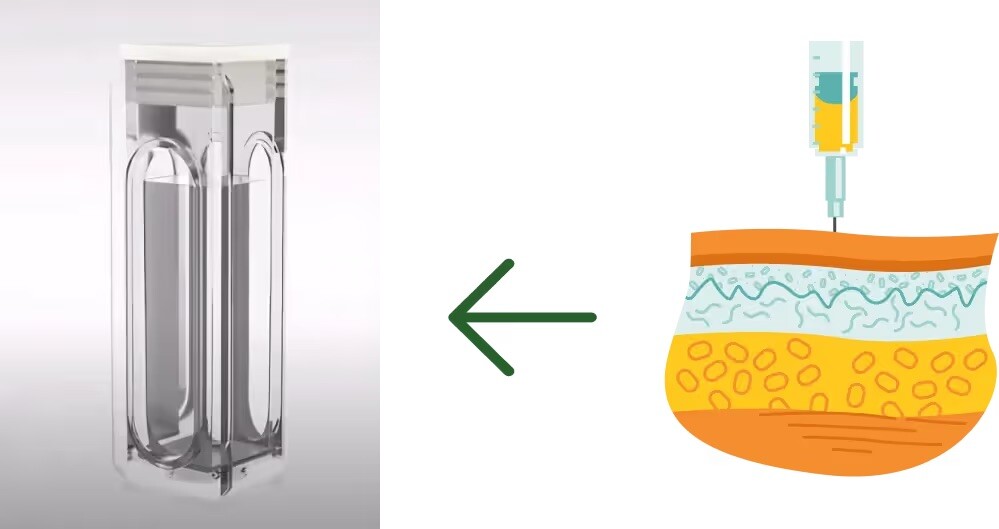
To simulate the injection site, a cartridge containing two customized dialysis membranes is filled with materials that mimic the extracellular matrix (ECM) composition of the subcutaneous tissue. This is suspended within a chamber containing a bicarbonate-based buffer bath which acts as a sink, representing the systemic circulation.
A subcutaneous formulation is injected into this simulated injection site/cartridge and the optical density at the injection site is monitored to detect possible instabilities such as precipitation/aggregation upon injection. A pH probe is also placed in the simulated injection site to gain information on how the pH of the ECM is affected by the injection of formulation, and how long it takes the injection site to return to homeostasis following injection. This data can be used to infer whether the instabilities are related to a particular pH.
Concentration is measured directly in the biorelevant bicarbonate-based buffer to determine time-concentration profiles of biopharmaceutical and formulation components that have diffused out of the simulated injection site, through the dialysis membranes, into systemic circulation over time.

Pion’s quick response time to our service needs keeps downtime to a minimum in our lab.
QC Director
Tennessee
They truly care about the customer experience and the financial impacts on our company.
Company President
Florida
Pion’s service is the gold standard for our industry. They are in a league by themselves!
QC Supervisor
North Carolina